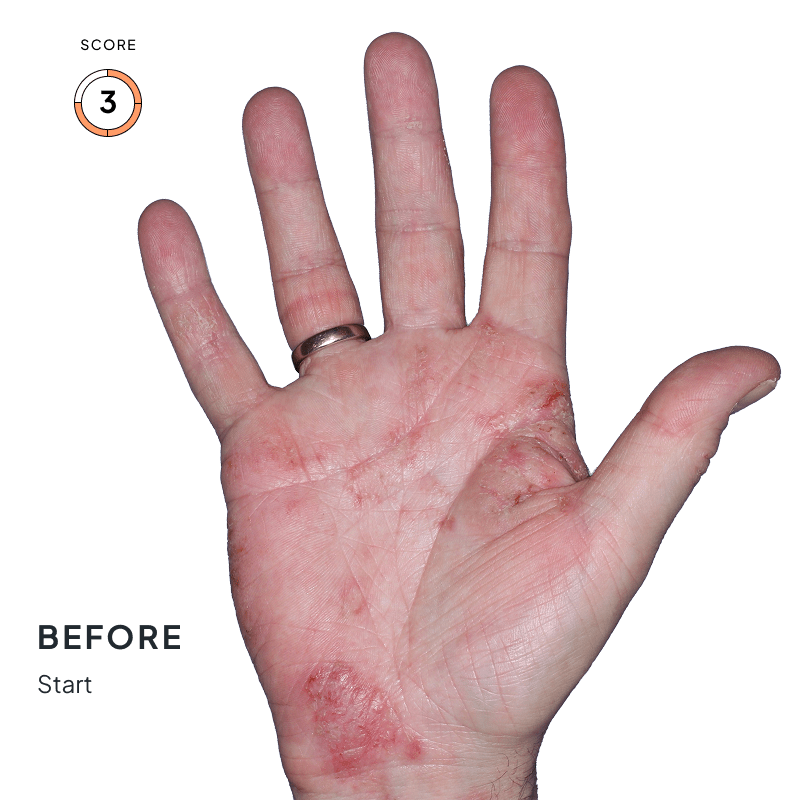
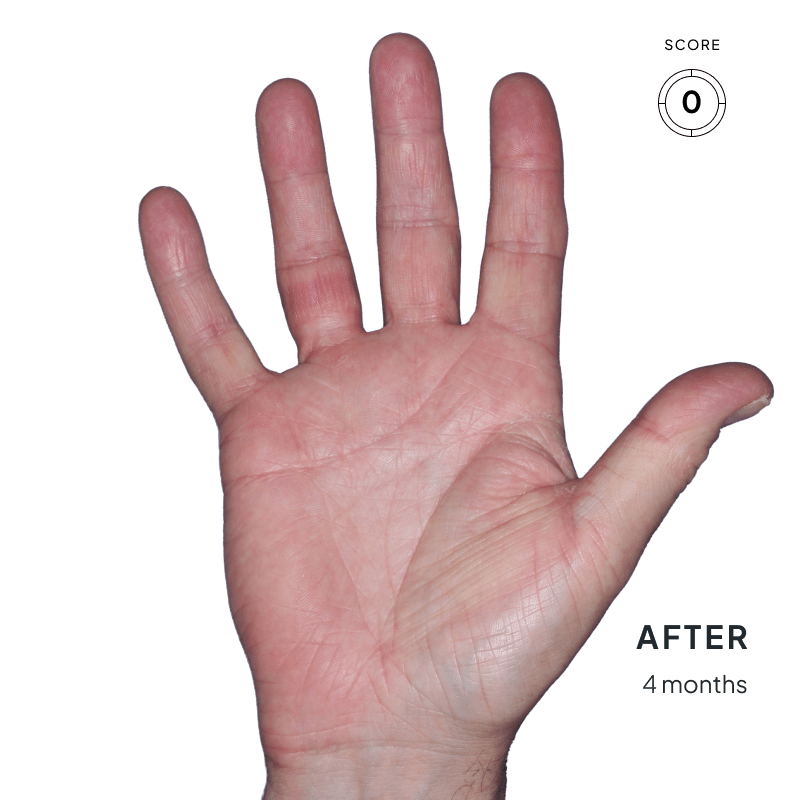
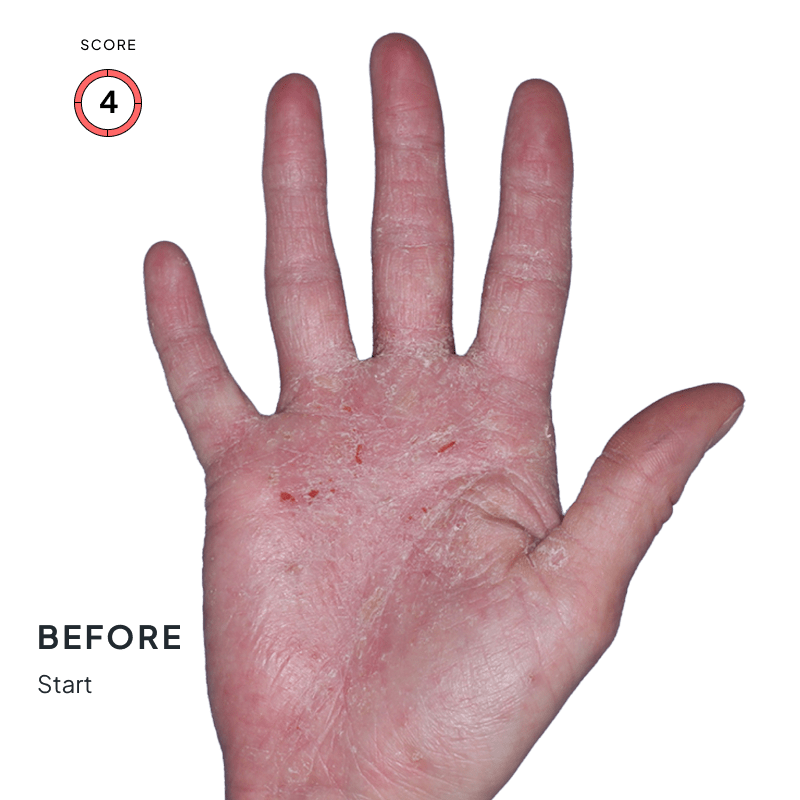
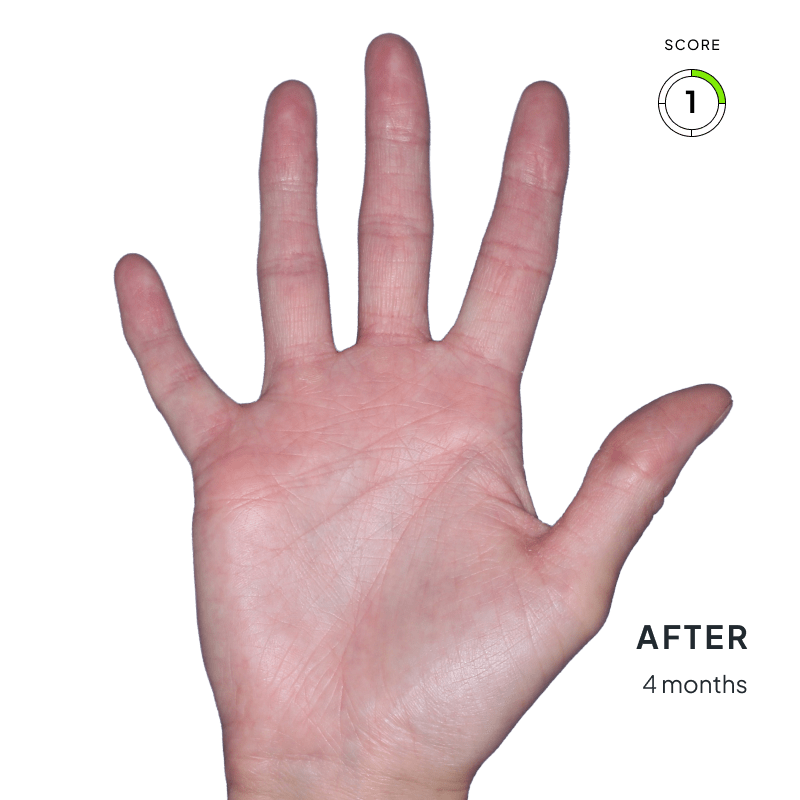
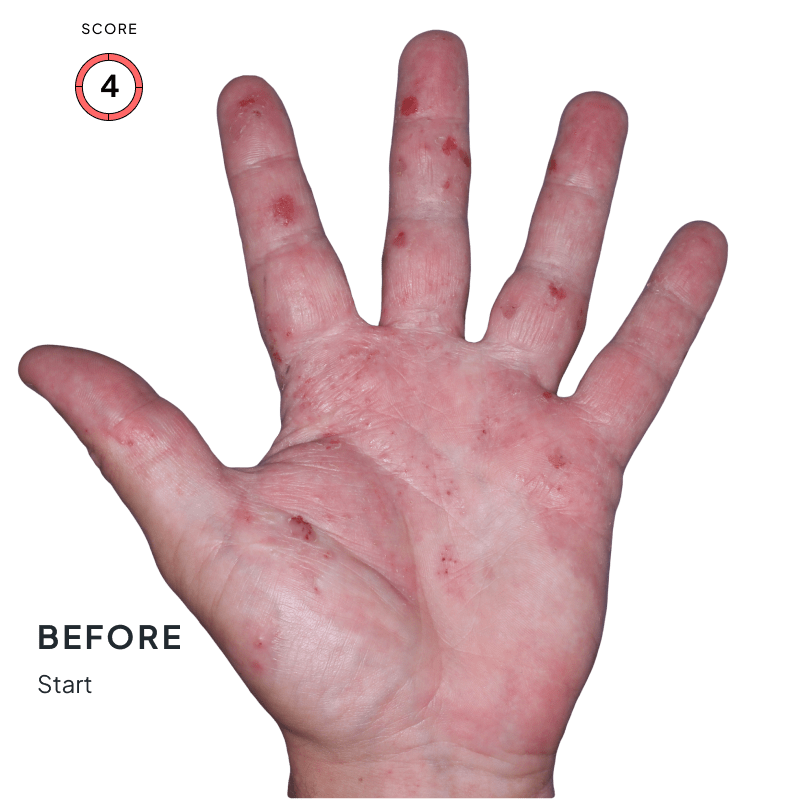
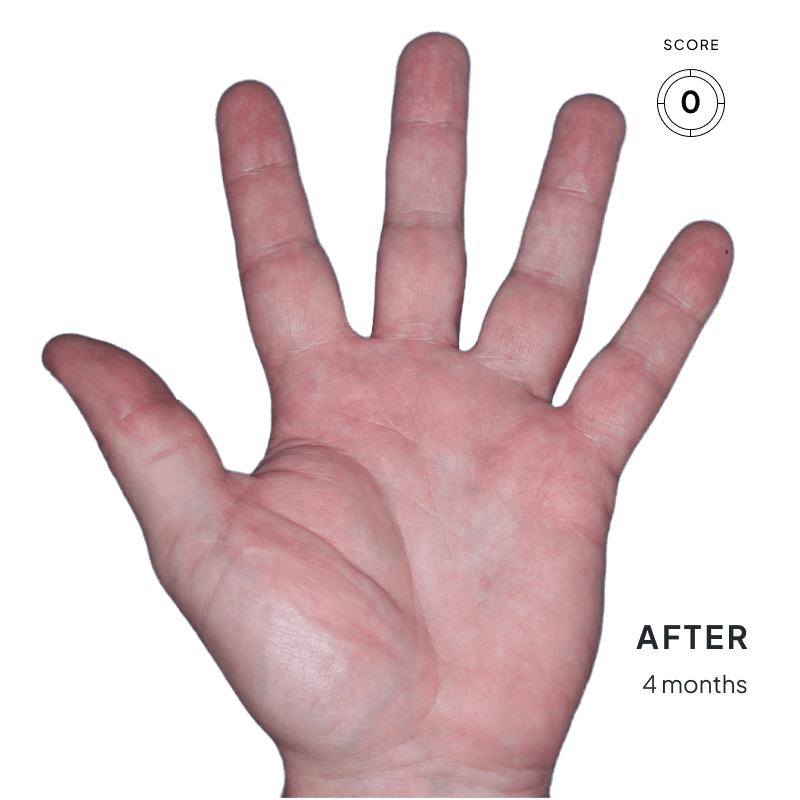
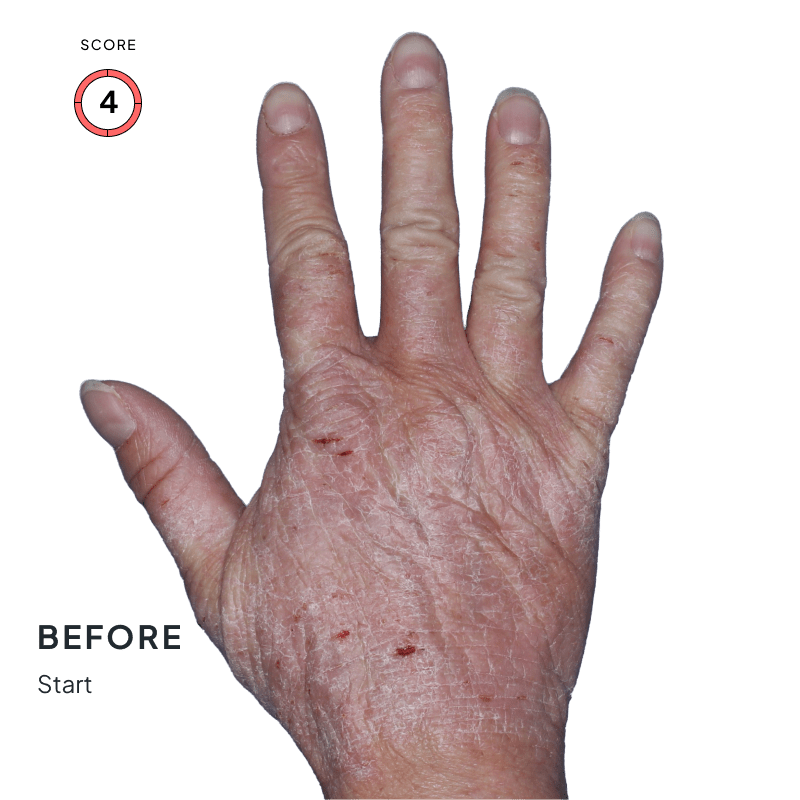
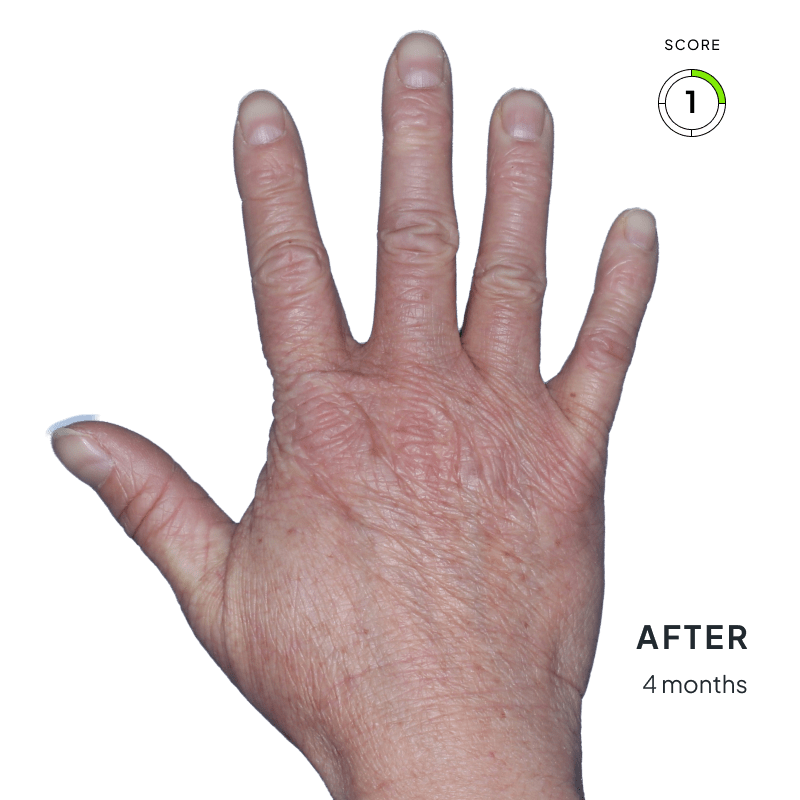
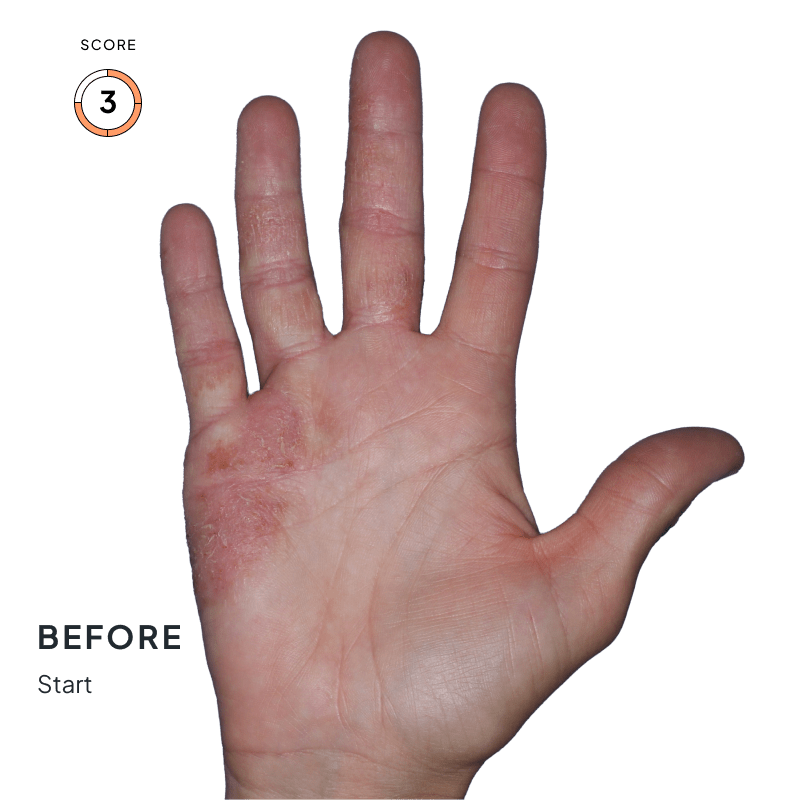
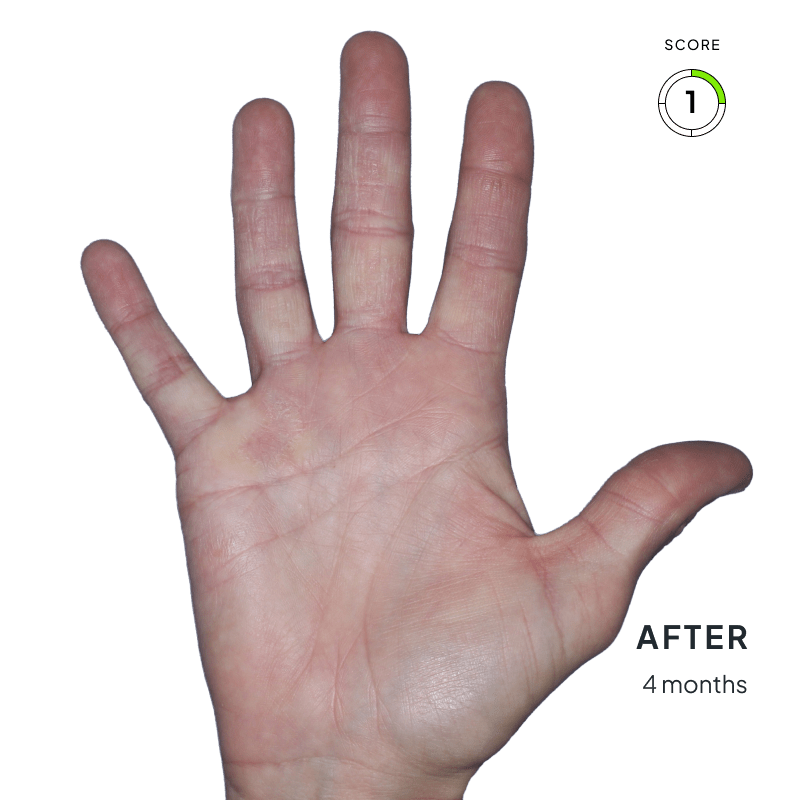
ANZUPGO is for use on the skin (topical use) only. Do not use ANZUPGO in or on your eyes, mouth, or vagina.
What is the most important information I should know about ANZUPGO?
ANZUPGO may cause serious side effects, including:
Serious Infections: ANZUPGO may increase your risk of infections. ANZUPGO contains delgocitinib. Delgocitinib belongs to a class of medicines called Janus kinase (JAK) inhibitors. JAK inhibitors are medicines that affect your immune system. JAK inhibitors can lower the ability of your immune system to fight infections. Some people have had serious infections while taking JAK inhibitors by mouth or applying on the skin, including tuberculosis (TB), and infections caused by bacteria, fungi, or viruses that can spread throughout the body. Some people have been hospitalized or died from these infections.
- ANZUPGO should not be used in people with an active, serious infection. You should not start using ANZUPGO if you have any kind of infection unless your healthcare provider tells you it is okay. You may be at a higher risk of developing shingles (herpes zoster) or eczema herpeticum (a blistery, painful skin rash) during treatment with ANZUPGO.
Before starting ANZUPGO, tell your healthcare provider if you:
- are being treated for an infection or have an infection that does not go away or that keeps coming back
- have TB or have been in close contact with someone with TB
- have had shingles (herpes zoster)
- have had hepatitis B or C
- think you have an infection or have symptoms of an infection such as fever, sweating, or chills; muscle aches; cough or shortness of breath; blood in your phlegm; weight loss; warm, red, or painful skin or sores on your body; diarrhea or stomach pain; burning when you urinate or urinating more often than usual; and/or feeling very tired
After starting ANZUPGO, call your healthcare provider right away if you have any symptoms of an infection. ANZUPGO can make you more likely to get infections or make worse any infections that you have. If you get a serious infection, your healthcare provider may stop your treatment with ANZUPGO until your infection is controlled.
Non-melanoma skin cancer. ANZUPGO may increase your risk of certain non-melanoma skin cancers. Your healthcare provider will regularly check your skin during your treatment with ANZUPGO.
- Avoid sunlamps and limit the amount of time you spend in the sunlight. Wear protective clothing when you are in the sun, and use a broad-spectrum sunscreen
- Tell your healthcare provider if you have ever had any type of cancer
Potential risks from Janus kinase (JAK) inhibition. It is not known whether using ANZUPGO has the same risks as taking oral or other topical JAK inhibitors. Increased risk of death (all causes) has happened in people who were 50 years of age and older with at least one heart disease (cardiovascular) risk factor who were taking a JAK inhibitor used to treat rheumatoid arthritis (RA) compared to people taking another medicine in a class of medicines called TNF blockers. ANZUPGO is not for use in people with RA. Oral or other topical JAK inhibitors have also caused increased cholesterol.
Before using ANZUPGO, tell your healthcare provider about all your medical conditions, including if you:
- have an infection
- have recently received or are scheduled to receive a vaccine. People who use ANZUPGO should not receive live vaccines right before starting, during treatment, or right after treatment with ANZUPGO
- are pregnant or plan to become pregnant. It is not known if ANZUPGO will harm your unborn baby
- are breastfeeding or plan to breastfeed. It is not known if ANZUPGO passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with ANZUPGO. If you use ANZUPGO while breastfeeding, avoid touching the nipple and surrounding area right away after applying ANZUPGO to your hands and wrists
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
What are the most common side effects of ANZUPGO?
- application site reactions, including pain, tingling, itching, and redness; bacterial skin infections, including finger cellulitis and nail infections; and low white blood cells
These are not all of the possible side effects of ANZUPGO. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Please see full Prescribing Information and Medication Guide.