AT 4 MONTHS:
UP TO
4X MORE
PEOPLE
SAW CLEAR OR ALMOST-CLEAR
SKIN
ANZUPGO vs
non-medicated cream
- Study 1: 20% vs 10%
- Study 2: 29% vs 7%
In Study 1: 325 people used ANZUPGO, and 162 people used the non-medicated cream. In Study 2: 313 people used ANZUPGO, and 159 people used the non-medicated cream.
MORE THAN
2X AS MANY
PEOPLE
HAD SIGNIFICANT ITCH RELIEF
ANZUPGO vs
non-medicated cream
- Study 1: 47% vs 23%
- Study 2: 47% vs 20%
In Study 1: 323 people used ANZUPGO, and 161 people used the non-medicated cream. In Study 2: 309 people used ANZUPGO, and 156 people used the non-medicated cream.
UP TO
2X MORE
PEOPLE
HAD SIGNIFICANT PAIN RELIEF
ANZUPGO vs
non-medicated cream
- Study 1: 49% vs 28%
- Study 2: 49% vs 23%
In Study 1: 291 people used ANZUPGO, and 149 people used the non-medicated cream. In Study 2: 294 people used ANZUPGO, and 141 people used the non-medicated cream.
SIGNIFICANTLY CLEARER
SKIN AS EARLY AS 4 WEEKS
ANZUPGO vs non-medicated cream
- Study 1: 15% vs 5%
- Study 2: 15% vs 8%
In Study 1: 325 people used ANZUPGO, and 162 people used the non-medicated cream. In Study 2: 313 people used ANZUPGO, and 159 people used the non-medicated cream.
Actor portrayal.
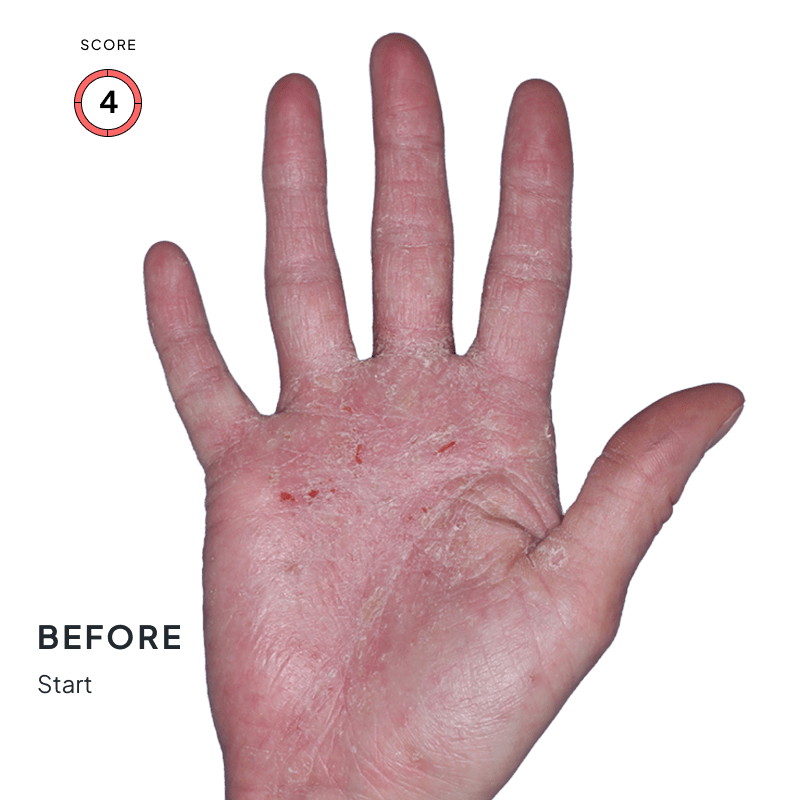
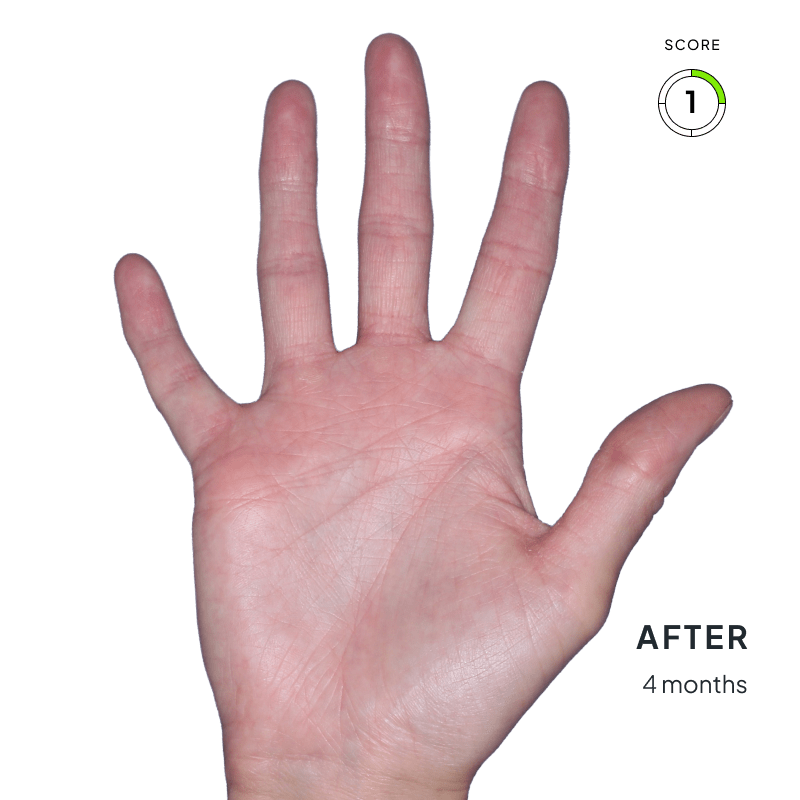
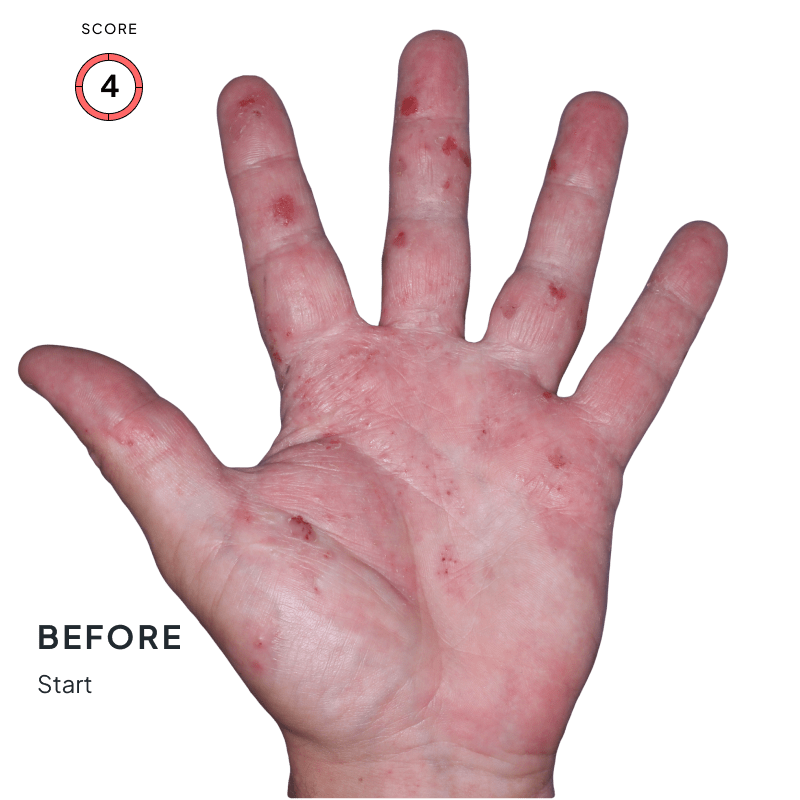
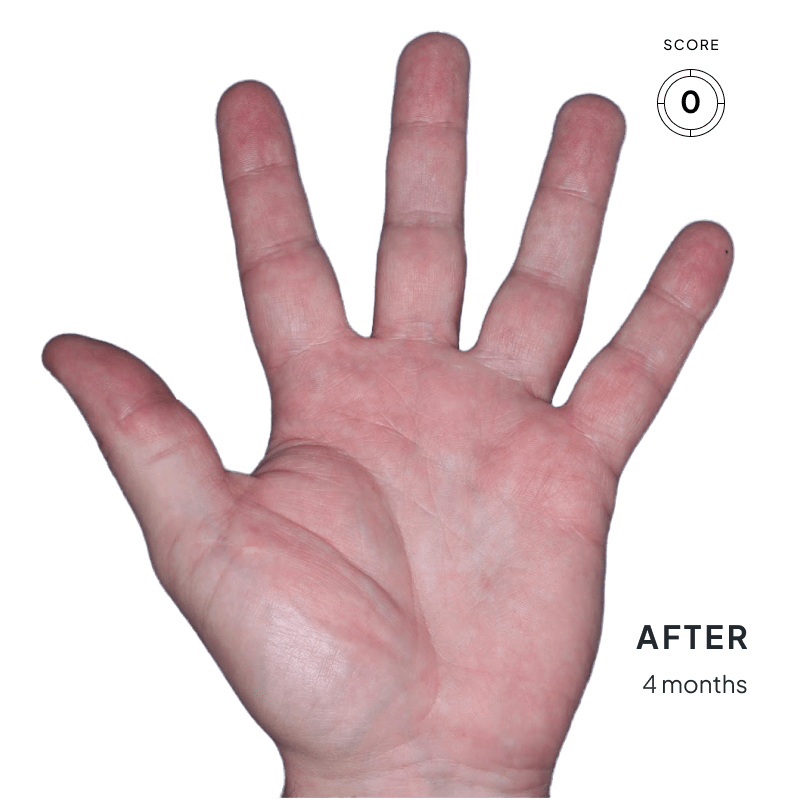
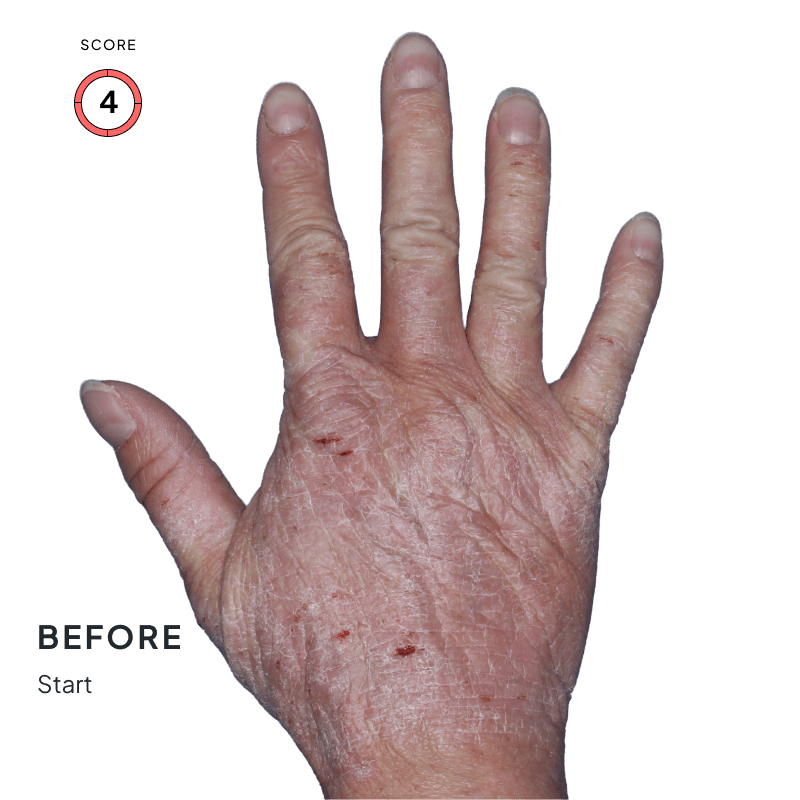
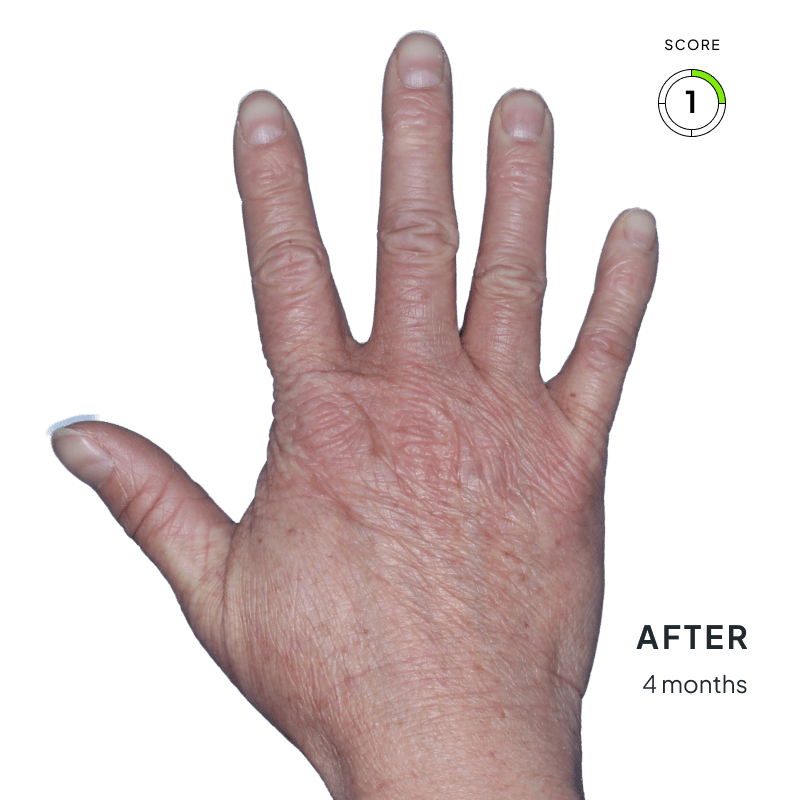
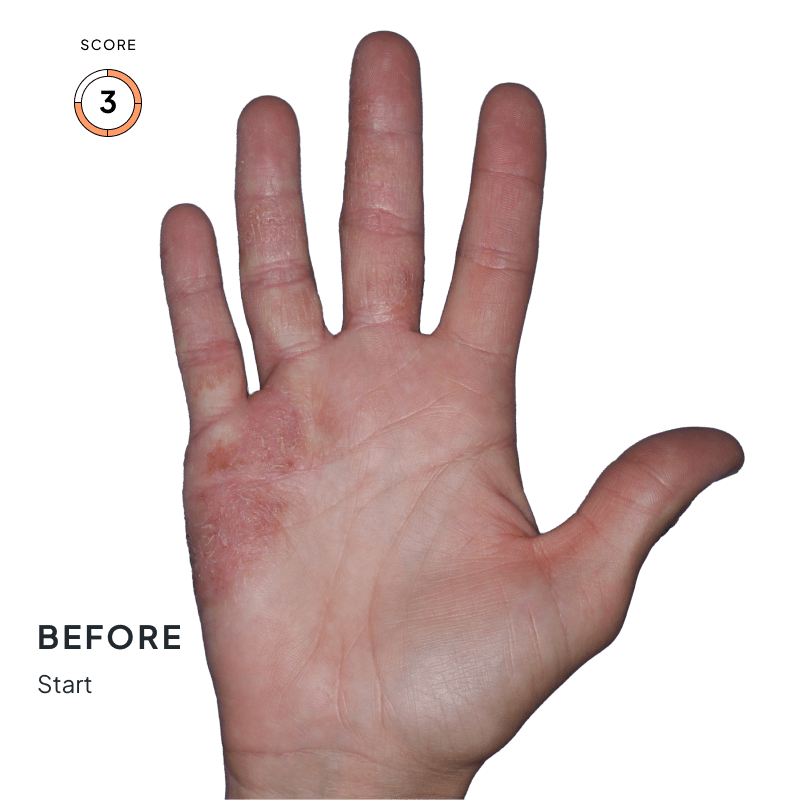
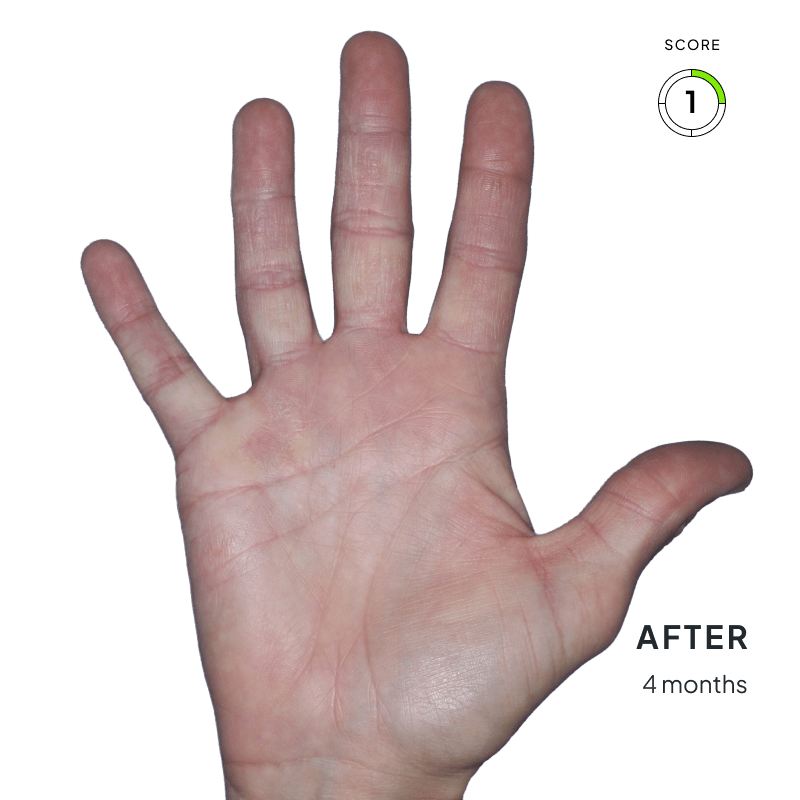
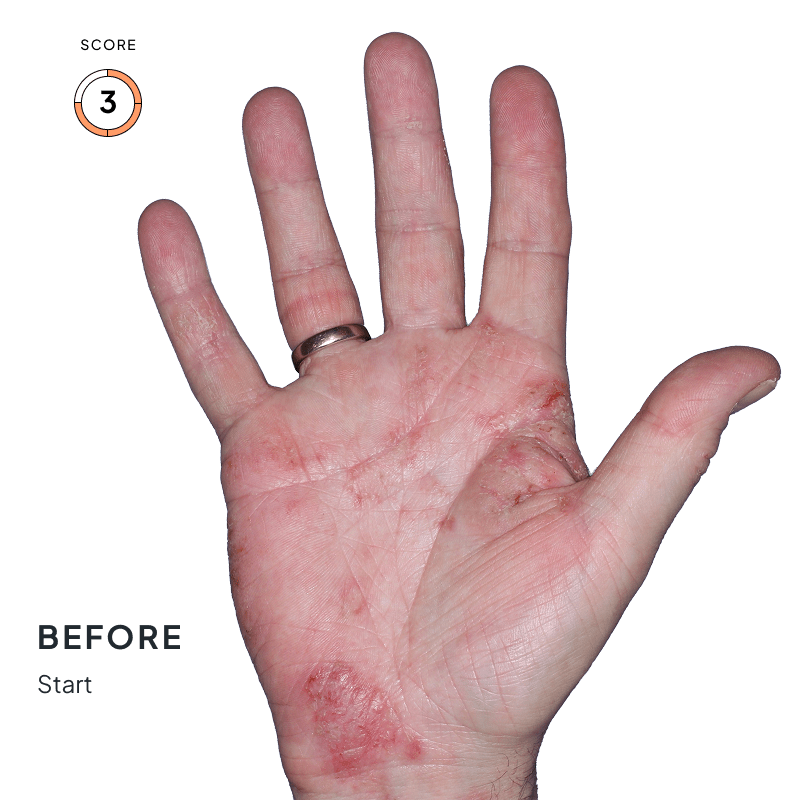
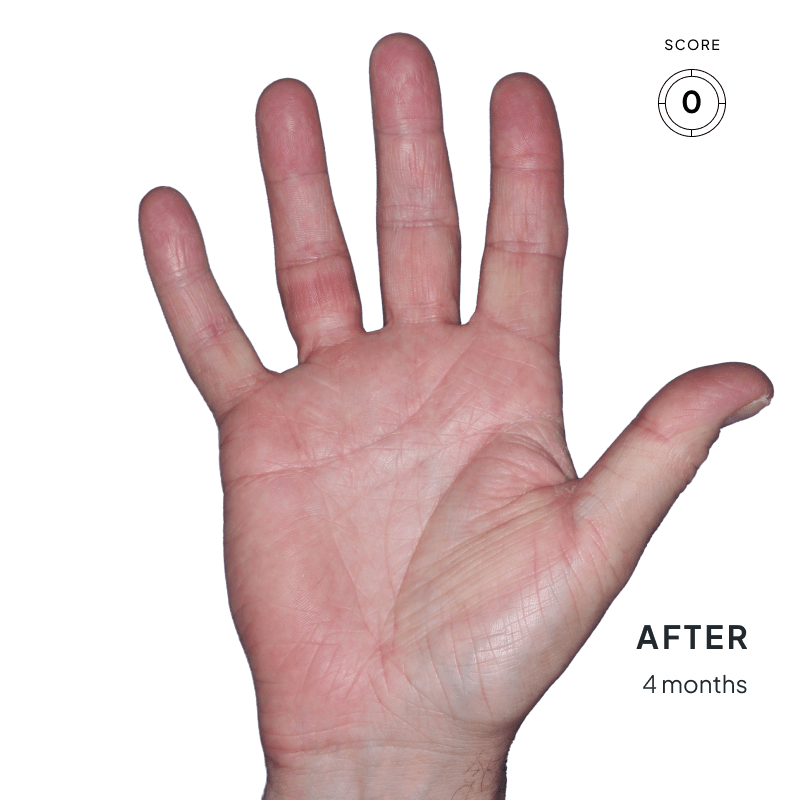
BEFORE & AFTER:
SEE THE DIFFERENCE
Explore photos from the ANZUPGO clinical studies here.
Throughout the studies, participants were given a score from 0 to 4.
- 0 = clear skin
- 4 = severe CHE
Success was defined as achieving both:
- An end score of 0 (clear) or 1 (almost-clear)
- At least a 2-point drop in score from the beginning of the study
CHE relief IN THEIR OWN WORDS
ANZUPGO helped many adults with moderate-to-severe CHE get relief from itch and pain.
ANZUPGO demonstrated safety profile
ANZUPGO demonstrated safety profile
In studies, the most common side effects reported in less than 1% of ANZUPGO treated patients include:
- application site reactions, including pain, tingling, itching and redness
- bacterial skin infections, including finger cellulitis, and nail infections
- low white blood cells
These are not all of the possible side effects of ANZUPGO.
The safety of ANZUPGO has been evaluated in a long-term clinical study for
UP TO 1 YEAR

Actor portrayal.